|
|
METODOLOGY
- AMPHIPODS
How to set an amphipod trap
 First, an unavailable bait (pork meet) was prepared. It was kept in a small perforated container which enables amphipods to smell it but not eat it (so we can find out in their stomach what they really eat in their natural environment).
First, an unavailable bait (pork meet) was prepared. It was kept in a small perforated container which enables amphipods to smell it but not eat it (so we can find out in their stomach what they really eat in their natural environment).
 The bait is put into the traps (they have a funnel at the end so amphipods can easily enter but not run away). We closed the other end of the trap with 0.5 mm net - later amphipod can be easily taken out.
The bait is put into the traps (they have a funnel at the end so amphipods can easily enter but not run away). We closed the other end of the trap with 0.5 mm net - later amphipod can be easily taken out.
 Traps were set into the water - three of them near the bottom, at the depth of 15 meters and one just under the ice cover. They were left it for 24 hours and next day emptied.
Traps were set into the water - three of them near the bottom, at the depth of 15 meters and one just under the ice cover. They were left it for 24 hours and next day emptied.
 All caught amphipods were put into the thermoses so they did not freeze during the way back and animals stayed alive until we got to the lab.
All caught amphipods were put into the thermoses so they did not freeze during the way back and animals stayed alive until we got to the lab.
 In the lab amphipods were identified and frozen in -80C degrees for stable isotopes and lipid analysis. Some were put in formalin - for further analyses of stomach content.
In the lab amphipods were identified and frozen in -80C degrees for stable isotopes and lipid analysis. Some were put in formalin - for further analyses of stomach content.
- BENTOS
How to collect benthic samples
 Benthos are the organisms which live on, in, or near the bottom. We sampled benthos with a petit ponar grab.
Benthos are the organisms which live on, in, or near the bottom. We sampled benthos with a petit ponar grab.
 Petit Ponar grab gives a whole new meaning to the word "petite." It's greatest advantage is the fact that it can be easily carried by one person in one hand. You may still need good muscle mass, but, since it weighs under 12 kg, you can use it on a line without the winch and crane. Its sample area is 152 x 152 mm. We used it to collect samples of bottom organisms on depth profile close to the glacier and near NyAlesund station.
Petit Ponar grab gives a whole new meaning to the word "petite." It's greatest advantage is the fact that it can be easily carried by one person in one hand. You may still need good muscle mass, but, since it weighs under 12 kg, you can use it on a line without the winch and crane. Its sample area is 152 x 152 mm. We used it to collect samples of bottom organisms on depth profile close to the glacier and near NyAlesund station.
 First we prepared grab for sampling. We opened it and blocked the lock. Than we launched it into the water. Once it hit the bottom it automatically unblock and grab some volume of the soft sediment.
First we prepared grab for sampling. We opened it and blocked the lock. Than we launched it into the water. Once it hit the bottom it automatically unblock and grab some volume of the soft sediment.
Once the sample was on the ice it was packed into the plastic bag and later into the coolers to protect samples from freezing.
 In the lab samples were sieved along 0.5 mm sieve to obtain all animals larger than the sieve size (macrobenthos) and clean them from sediment.
In the lab samples were sieved along 0.5 mm sieve to obtain all animals larger than the sieve size (macrobenthos) and clean them from sediment.
 To keep animals alive as long as possible we put them into the tanks with ambient water.
To keep animals alive as long as possible we put them into the tanks with ambient water.
 Than all animals are picked up but only alive ones were immediately identified and frozen for isotope and lipid analysis.
Than all animals are picked up but only alive ones were immediately identified and frozen for isotope and lipid analysis.
 All the others were preserved first in formaline than in 70% alcohol.
All the others were preserved first in formaline than in 70% alcohol.
- ZOOPLANKTON
Zooplankton - short guideline
Zooplankton are small animals living in almost all water bodies. They are certainly the most numerous animals on the Earth. They feed on phytoplankton and turn it effectively into own body mass. The zooplankter's body can consist of as much as 90% of lipids. Therefore, they are eagerly eaten by fish, birds and whales and by the amphipods.
 We sampled zooplankton with the WP2 net. Although we had two types of nets - 200um and 500um - the latter one appeared to more efficient in catching larger zooplankters. In order to obtain the sample, the net was lowered almost to the bottom and then pulled up to the surface.
We sampled zooplankton with the WP2 net. Although we had two types of nets - 200um and 500um - the latter one appeared to more efficient in catching larger zooplankters. In order to obtain the sample, the net was lowered almost to the bottom and then pulled up to the surface.

 Zooplankton which was suspended in the water column was eventually caught in the plastic cod end and then placed in the insulated plastic container in order to prevent it from freezing.
Zooplankton which was suspended in the water column was eventually caught in the plastic cod end and then placed in the insulated plastic container in order to prevent it from freezing.
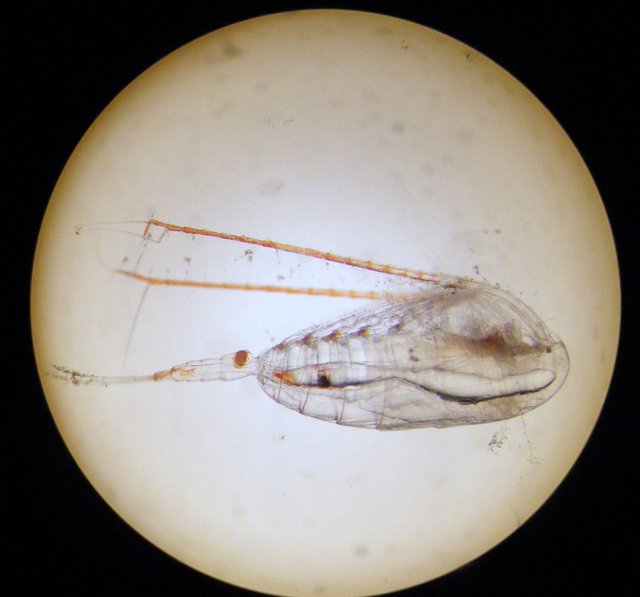 The first analysis of zooplankton revealed that the amphipods have an access to the Copepods (mainly Calanus glacialis), sea snails (Clione limacina) and arrow worms (Sagitta elegans).
The first analysis of zooplankton revealed that the amphipods have an access to the Copepods (mainly Calanus glacialis), sea snails (Clione limacina) and arrow worms (Sagitta elegans).
|